All humans die. However, while each of us knows we shall someday die, few of us can escape wishing we could delay the process. Some succeed. The oldest documented living person, Marie-Louise Febronie Meilleur of Corbeil, Ontario, reached the age of 117 years in 1997. The tantalizing possibility of long life that she represents is one reason why there is such interest in the aging processif we knew enough about it perhaps we could slow it. A wide variety of theories have been advanced to explain why we age. In the last year scientists have come a long way towards unraveling the puzzle.
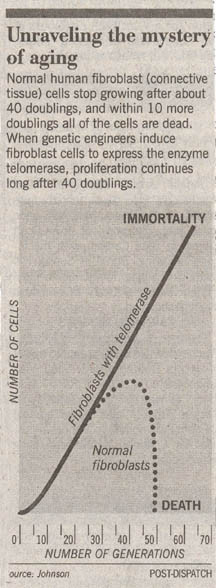
The first clue was the discovery that cells appear to die on schedule, as if following a script. In a famous experiment carried out in 1961, geneticist Leonard Hayflick demonstrated that fibroblast cells growing in tissue culture will divide only a certain number of times. After about 50 population doublings, cell division stops, the cell cycle blocked just before DNA replication. If a cell sample is taken after 20 doublings and frozen, when thawed it resumes growth for 30 more doublings, then stops.
An explanation of the “Hayflick limit” was suggested in 1986 when Howard Cooke first glimpsed an extra length of DNA at the ends of chromosomes. These telomeric regions, about 5,000 nucleotides (the chemical letters of DNA), are each composed of several thousand repeats of the sequence TTAGGG. Cooke found the telomeric region to be substantially shorter in body tissue chromosomes than in those of germ line cells, the egg and sperm. He speculated that in body cells a portion of the telomere cap was lost by a chromosome during each cycle of DNA replication.
Cooke was right. The cell machinery that replicates the DNA of each chromosome sits on the last 100 units of DNA at the chromosome’s tip, and so cannot copy that bit. So each time the cell divides, its chromosomes get a little shorter. Eventually, after some 50 replication cycles, the protective telomeric cap is used up, and the cell line then enters senescence, no longer able to proliferate.
How do sperm and egg cells avoid this trap, dividing continuously for decades? Scientists have recently learned that all human cells contain an enzyme, telomerase, which lengthens telomeres. This enzyme is active in sperm and egg cells, maintaining their chromosomes at a constant length of 5,000 nucleotide units. In body cells, by contrast, the telomerase gene is silent.
Research published last year has confirmed Cooke’s hypothesis, providing direct evidence for a causal relation between telomeric shortening and cell senescence. Using genetic engineering, teams of researchers from California and Texas transferred into human body cell cultures a DNA fragment that unleashes each cell’s telomerase gene. The result was unequivocal. New telomeric caps were added to the chromosomes of the cells, and the cells with the artificially elongated telomeres did not senesce at the Hayflick limit, continuing to divide in a healthy and vigorous manner for more than 20 additional generations.
This research shows clearly that loss of telomere DNA eventually restrains the ability of human cells to proliferate. And yet every human cell possesses a copy of the telomerase gene that, if expressed, would rebuild the telomere. Why do our cells accept aging, if they need not? The answer, it seems, is to avoid cancer. By limiting the number of divisions allotted to human cell lines, the body insures that no cell can continue to divide indefinitely. Suppression of the telomerase gene is, in a very real sense, cancer suppression. When scientists examine cancer cells, they commonly find their telomerase genes have been activated and are maintaining telomeres at full length. Thus telomere shortening is a tumor-suppressing mechanism, one of your body’s key safeguards against cancer.
Aging, then, is at least partly a strategy to avoid the inevitable consequence of wear and tear to our genes mutations that sooner or later lead to cancer. A powerful source of such mutations are free radicals, highly reactive fragments of atoms with unpaired electrons that sheer through DNA like a shotgun blast. Free radicals are an unavoidable byproduct of how our bodies use oxygen to metabolize food. Because free radicals are so distructive, every cell has numerous mechanisms to control and eliminate them. If all else fails to eliminate free radicals, the cell activates a second key dafeguard against cancer. A special fail-safe gene called p66 pulls the plug, causing the cell to commit suicide rather than permit free radicals to damage the DNA and produce cancer.
Work published in November by Italian researchers indicates that mice with disabled p66 genes live longer than normal mice. Their cells were no longer being zapped by the hair-trigger p66 self-distruct mechanism. Inhibitory drugs of the p66 class of protein are known. If you are willing to accept the added risk of cancer, prehaps taking them would, like the Italian mice, let you live longer. ©Txtwriter Inc.