Exciting New Evidence Points the Way to How We Learn
This month marks a hallmark in the history of biology. For the first time, we understand how learning takes place. Not vaguely, with ill-defined concepts and analogies, but in concrete precise molecular detail. The discovery of the molecular basis of learning is likely to impact science far into the future, just as the discovery of DNA’s structure in 1952 by Watson and Crick opened new vistas scarcely imagined then.
Like the discovery of DNA, and indeed like most advances in science, this discovery had its beginnings in the work of others, and is but the latest chapter in a long history of investigation. For all practical purposes, the story starts fifty years ago, when a prominent Canadian psychologist named Donald Hebb set out to summarize everything then known about how the brain works to produce behavior. Deep within his treatise, The Organization of Behavior, Hebb proposed an original and deceptively simple hypothesis about the nature of learning.
It was already known in 1949 that long-term memory — the basis of associative learning — is centered in the forebrain, and deep within the base of the brain in a region called the hippocampus. Many scientists believed then that memory was some sort of electrical “field effect” shared by many cells. Hebb would have none of this. Instead, he started with the assumption that the changes involved in learning occur at the level of individual nerve cells within the forebrain and hippocampus. Learning, Hebb argued, must involve changes in the way these nerve cells link up with each other. Hebb went on to propose a simple way in which nerve connections could be altered to produce learning. He had little experimental data to guide him, but argued that the scheme he proposed was the most logical way to do it.
For the last fifty years neurobiologists have explored Hebb’s idea, gradually learning more and more about how the brain learns and remembers. This month a team of researchers led by Dr. Joe Tsien, a young assistant professor at Princeton University, proved Hebb right, and in the process opened the door to a Pandora’s box of exciting — and frightening — possibility.
To understand what Hebb proposed, and Tsien confirmed, it is necessary to step back for a moment and looks at how our brains process information — how it’s nerve cells talk to one another.
For a start, they are not wired like a computer. In a computer, the wires are all welded together, making direct electrical contact. In your brain, the nerve cells don’t actually touch each other, although they get very close. Understanding what happens in the tiny gap that remains between them is the secret to Hebb’s proposal, and Tsien’s discovery.
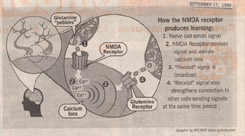
When a nerve cell in the brain’s hippocampus wants to send a signal across the gap to a neighbor nerve cell, it does it like a little boy might, by throwing pebbles. Each pebble is a kind of chemical called a neurotransmitter. In your brain the neurotransmitter is the amino acid glutamine. On the receiving side of the gap are a cluster of targets for the pebbles, each in effect a tiny electrical switch.
When a pebble hits one of these switches, called a receptor, it reacts with a molecular “ouch,” opening up a channel into the receiving cell. The channel is only open the briefest of moments — a few hundred thousandths of a second — but that’s long enough to let in a hoard of sodium ions massed outside. Because sodium ions carry positive charges, their entrance produces a local electrical disturbance, a submicroscopic thunderstorm that in turn opens other nearby channels, which open other channels. A chain reaction of electrical disturbance spreads out, sending the signal racing off along the surface of the receiving nerve cell.
Hebb proposed that when a receptor in the forebrain or hippocampus is hit by a glutamine neurotransmitter, something else happens too. Not only is the signal received and passed along, but an acknowledgment is sent back, like a receipt. A chemical returns to the nerve cell that threw the pebble, saying, in effect, “message received.” This receipt strengthens the connection between the two nerve cells, making future communication easier.
Now here’s the key point: ANY nerve cell junction in the neighborhood that happened to be firing out pebbles at just that time gets strengthened — even ones that had nothing to do with the original message. Thus nerve cells that fire at the same time tend to establish firm junctions with one another, while nerve cells that fire out-of-synch don’t.
This process of linking together nerves that fire at the same time is called “association.” When the nerve signals representing the sound of your mother’s voice pass through the hippocampus at the same time as the nerve signals representing the image of her face. You “learn” to associate the sound with the image. Hebb proposed that links between nerve cells are stabilized by simultaneous activity, leading to associative learning.
If you think about it, the sort of receptor Hebb proposed as the basis of memory has two jobs to do: not only must it link up nerve cells that tend to fire at the same time, it must also forge stronger links for stronger signals. This means it must be able to detect when other pebbles are impacting nearby.
When molecular biologists examined the typical glutamine receptors on the nerve cells of the forebrain and hippocampus, it was hard to see how they could perform both tasks. They are excellent “pebble targets’ — great at sending signals along — but too simple for more complex work. Besides, chemicals that block memory don’t affect them.
There is one special kind of glutamine receptor on these cells, however, that seems a more likely candidate. Called an NMDA receptor (for the arcane chemical reason that it also responds to a modified form of glutamine called N-Metheyl-D-Aspartate), this receptor has the unusual property that it requires TWO different kinds of inputs before it will open its channel. Not only must it get hit by a glutamine pebble, but at the same time it must be perturbed by a localized electrical disturbance (that is, another pebble must have hit nearby).
When an NMDA receptor does open up, it lets in not only sodium ions like the other glutamine receptors, but also calcium ions. These calcium ions act like a “call to arms” when they pour into the nerve cell that has been hit by the pebble, initiating a whole series of changes. In a variety of ways, these changes strengthen the nerve cell’s connection to the pebble thrower. Importantly among them is the broadcast back to the pebble thrower of the “receipt” signal. It is this return signal (not as yet isolated by neurochemists) that Hebb suggested is responsible for forging associations with other nerve signal paths firing at the same time. Stronger signals form stronger links: the more times NMDA receptors are fired, the more calcium gets in and the stronger the broadcast that forms associations.
The NMDA receptor is thus a very attractive candidate for Hebb’s learning receptor — its even influenced by memory inhibitors. But because a picture is pretty doesn’t mean that the picture is correct. Seeing the possibilities, however, many scientists set out to study the NMDA receptor. Perhaps knowing more about it might suggest a test of Hebb’s hypothesis.
The NMDA receptor proved to be a complicated molecular machine, sort of a manhole in the cell’s surface with a trap door that can be swung open. The NMDA receptor is built of a variety of protein subunits — imagine making a tunnel with a bunch of pencils. Some of the protein “pencils” are involved in snapping open the trap door, others prevent this unless they detect local electrical disturbance, still others keep the door closed until a glutamine “pebble” has hit the receptor. When the trap door is open, yet another set of protein subunits acts as a tunnel or channel for calcium ions to enter the nerve cell.
It is on this last kind of subunit that Tsien has focused. One version, called NR2B, is common among the NMDA receptors of young people and lets in a lot of calcium ions. A second version, which tends to replace NR2B as we grow older, is called NR2A and lets in far less calcium. If Hebb is right, then NR2B-rich NMDA receptors should be much better at establishing nerve cell connections — and at promoting learning! This would explain why younger animals learn so much faster than older ones.
It is this specific prediction of Hebb’s hypothesis that Tsien and his coworkers set out to test. They created special strains of mice that had extra copies of the gene encoding the NR2B subunit, and modified the genes so that they would only be expressed in the forebrain and hippocampus.
When the research team isolated and examined nerve cells from the forebrain and hippocampus of their NR2B-enriched mice, they found that the NMDA receptors were in deed admitting elevated amounts of calcium. Instead of staying open for 100 thousandths of a second, as normal receptors do, the channels of these receptors were remaining open for 250 thousandths of a second, time to admit over twice as much calcium. This told Tsien that he had set up a fair test, that he had altered the behavior of the NMDA receptors in the intended way.
Now comes the true test. Are the mice able to learn faster, as Hebb’s hypothesis predicts? Has Tsien created “smarter” mice?
To carry out the test of learning ability, Tsien and his coworkers ran the NR2B-enriched mice through a battery of six standard learning tests, designed to use different association areas of the mouse forebrain and hippocampus. Some of the tests required the mice to learn to recognize unfamiliar objects, others to remember how to find a hidden platform when dropped into murky water. In all of the tests, the genetically souped-up mice did better than normal mice. Time and again, for each test, NR2B-enriched mice performed consistently better.
Because the six tests were chosen to involve many different parts of the forebrain and hippocampus, Tsien could be confident that the result was general — that the mice really were smarter, and hadn’t just gained the ability to perform a particular task better. Adding more NR2B subunits to their NMDA receptors had indeed improved the basic mechanism of their memory, just as Hebb’s hypothesis predicts.
Hebb was also right in a more fundamental way — learning is the result of molecular interactions that take place between nerve cells in the brain. Intelligence? For these mice, it was a matter of a delay of 150 thousandths of a second in how fast a protein receptor in their brain snapped shut.
Will we be able to take NR2B pills and get smarter? Not anytime soon. That’s not the importance of Tsien’s discovery. The point is that we now know how to ask specific concrete questions about learning. Psychology, neurobiology, and molecular biology — all converge in this new terrain into which Tsien has led us. As scientists we have just read the first page of what is probably a very long book, and know very little so far — BUT NOW WE KNOW HOW TO READ THE BOOK!
It may be quite a while before we gain the clinical ability to aid the memory-impaired, but I have little doubt that eventually this will become possible. Can we make people smarter? If the blueprint of how we think is written in the genes, will gene technology allow us in the future to alter this most basic of human abilities? While we can’t help asking this question, we don’t know the answer because we haven’t read the book yet. But we’ve started.
©Txtwriter Inc.