Life Cycle of the AIDS Virus
The virus responsible for the comparatively new and fatal viral disease, acquired immunodeficiency syndrome (AIDS). AIDS was first reported in the United States in 1981. It was not long before the infectious agent, a retrovirus (an RNA virus that transcribes its genes into DNA) called human immunodeficiency virus (HIV), was identified by laboratories in France. Study of HIV revealed it to be closely related to a chimpanzee virus, suggesting a recent host expansion to humans in central Africa from chimpanzees.
Infected humans have little resistance to infection, and nearly all of them eventually die of diseases that noninfected individuals easily ward off. Few who contract AIDS survive more than a few years untreated.
How HIV Compromises the Immune System
In normal individuals, an army of specialized cells patrols the bloodstream, attacking and destroying any invading bacteria or viruses. In AIDS patients, this army of defenders is vanquished. One special kind of white blood cell, called a CD4+ T cell is required to rouse the defending cells to action. In AIDS patients, the virus homes in on CD4+ T cells, infecting and killing them until none are left. Without these crucial immune system cells, the body cannot mount a defense against invading bacteria or viruses. AIDS patients die of infections that a healthy person could fight off.
Clinical symptoms typically do not begin to develop until after a long latency period, generally 8 to 10 years after the initial infection with HIV. During this long interval, carriers of HIV have no clinical symptoms but are apparently fully infectious, which makes the spread of HIV very difficult to control. The reason why HIV remains hidden for so long seems to be that its infection cycle continues throughout the eight- to 10-year latent period without doing serious harm to the infected person. Eventually, however, a random mutational event in the virus allows it to quickly overcome the immune defense, starting AIDS.
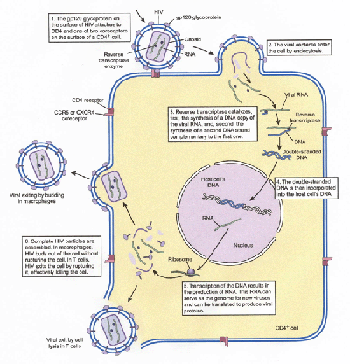
The HIV Infection Cycle
The HIV virus infects and eliminates key cells of the immune system, destroying the bodys ability to defend itself from cancer and infection. The way HIV infects humans (figure 4) provides a good example of how animal viruses replicate. Most other viral infections follow a similar course, although the details of entry and replication differ in individual cases.
Figure 4
The HIV infection cycle. The cycle begins and ends with free HIV particles present in the bloodstream of its human host. These free viruses infect white blood cells that have CD4 receptors, cells called CD4+ cells.
Attachment. When HIV is introduced into the human bloodstream, the virus particle circulates throughout the entire body but will only infect CD4+ cells. Most other animal viruses are similarly narrow in their requirements; hepatitis goes only to the liver, and rabies to the brain.
How does a virus such as HIV recognize a specific kind of target cell? Every kind of cell in the human body has a specific array of cell-surface glycoprotein markers that serve to identify them to other, similar cells. Each HIV particle possesses a glycoprotein (called gp120) on its surface that precisely fits a cell-surface marker protein called CD4 on the surfaces of immune system cells called macrophages and T cells. Macrophages are infected first.
Entry into Macrophages. After docking onto the CD4 receptor of a macrophage, HIV requires a second macrophage receptor, called CCR5, to pull itself across the cell membrane. After gp120 binds to CD4, it goes through a conformational change that allows it to bind to CCR5. The current model suggests that after the conformational change, the second receptor passes the gp120-CD4 complex through the cell membrane, triggering passage of the contents of the HIV virus into the cell by endocytosis, with the cell membrane folding inward to form a deep cavity around the virus.
Replication. Once inside the macrophage, the HIV particle sheds its protective coat. This leaves virus RNA floating in the cytoplasm, along with a virus enzyme that was also within the virus shell. This enzyme, called reverse transcriptase, synthesizes a double strand of DNA complementary to the virus RNA, often making mistakes and so introducing new mutations. This double-stranded DNA may integrate itself into the host cells DNA; it directs the host cell machinery to produce many copies of the virus. HIV does not rupture and kill the macrophage cells it infects. Instead, the new viruses are released from the cell by budding, a process much like exocytosis. HIV synthesizes large numbers of viruses in this way, challenging the immune system over a period of years.
Entry into T Cells. During this time, HIV is constantly replicating and mutating. Eventually, by chance, HIV alters the gene for gp120 in a way that causes the gp120 protein to change its second-receptor allegiance. This new form of gp120 protein prefers to bind instead to a different second receptor, CXCR4, a receptor that occurs on the surface of T lymphocyte CD4+ cells. Soon the bodys T lymphocytes become infected with HIV. This has deadly consequences, as new viruses exit the cell by rupturing the cell, thus killing the T cell. Thus, the shift to the CXCR4 second receptor is followed swiftly by a steep drop in the number of T cells. This destruction of the bodys T cells blocks the immune response and leads directly to the onset of AIDS, with cancers and opportunistic infections free to invade the defenseless body.
HIV, the virus that causes AIDS, is an RNA virus that replicates inside human cells by first making a DNA copy of itself. It is only able to gain entrance to those cells possessing a particular cell surface marker recognized by a glycoprotein on its own surface.